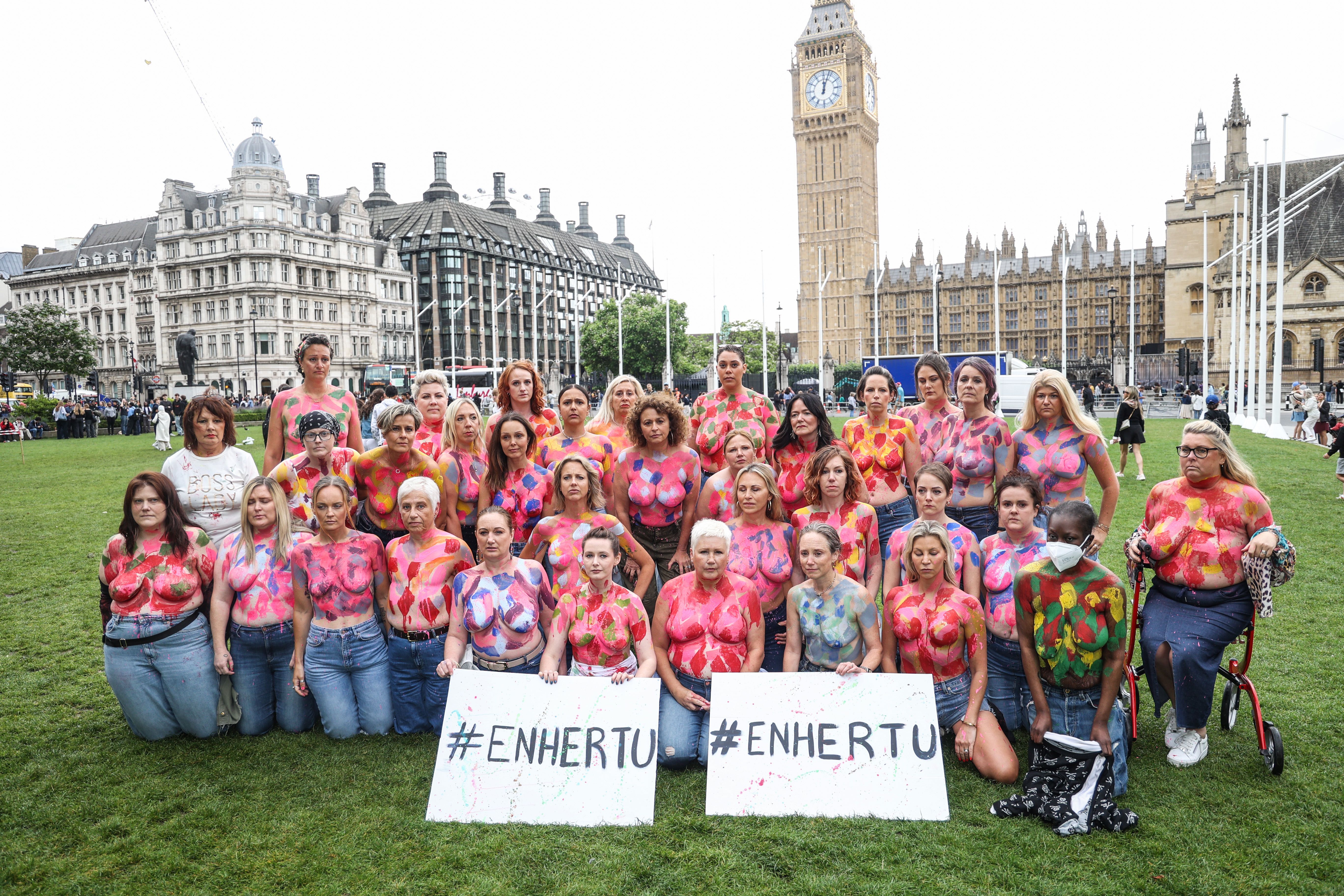A LAST-DITCH breast cancer drug has been formally rejected by the NHS watchdog due to its asking price.
In clinical trials Enhertu, known as trastuzumab deruxtecan, added months or even years to the lives of women with incurable breast cancer that had spread.
Health chiefs have squabbled with manufacturers for months over the cost of the drug.
The National Institute for Health and Care Excellence on Monday issued a final rejection and said the pharmaceutical companies were “unwilling to offer a fair price to the NHS”.
Its makers, Daichii Sankyo and AstraZeneca, said they had offered a secret deal cheaper than what the NHS in Scotland pays.
They say a change to England’s value for money sums has short-changed patients.
We are angry that women’s lives will be shortened as a direct result of this
Rachael Franklin
Campaigners including the charity Breast Cancer Now and Loose Women’s Nadia Sawalha are furious about the ruling.
They reckon 1,000 more women a year could benefit from the medicine if it were approved for HER2-low secondary breast cancer.
Loose Women’s Nadia, 59, said in a social media post: “It’s just heartbreaking.
“These are not just patients, they are human beings.”
Rachael Franklin, chief of Breast Cancer Now, said: “We are both devastated and angry that womens’ lives will be shortened as a direct result of this failure to agree a solution.
“Patients have found themselves in the middle of a stand-off about cost and the system which is denying them precious hope of more time to live.
“With Enhertu available to women in so many other countries, including Scotland, it is unacceptable that it will be out of reach for women in England, Wales and Northern Ireland.
“We will do all in our power to see this decision reversed.”
Blame game between two sides
A spokesman for Daichii Sankyo said: “We are very disappointed by and disagree with the decision NICE has made.
“It misclassifies HER2-low metastatic breast cancer as a ‘medium severity’ disease and this stands in the way of patient access.
“We remain committed to working with NICE to find a way forward.”
Helen Knight, director of medicines evaluation at NICE, said: “We are deeply disappointed that we are unable to recommend Enhertu for use in the NHS for advanced HER2-low breast cancer.
“NICE and NHS England offered as much flexibility as possible, but the companies did not put forward a new price, so we have no choice.
“I would like to thank the breast cancer community for their hard work on this issue and I am sorry we do not have better news.”
What are the signs of breast cancer?
BREAST cancer is the most common type of cancer in the UK.
The majority of women who get it are over 50, but younger women and, in rare cases, men can also get breast cancer.
If it’s treated early enough, breast cancer can be prevented from spreading to other parts of the body.
Breast cancer can have a number of symptoms, but the first noticeable symptom is usually a lump or area of thickened breast tissue.
Most breast lumps aren’t cancerous, but it’s always best to have them checked by your doctor. You should also speak to your GP if you notice any of the following:
- a change in the size or shape of one or both breasts
- discharge from either of your nipples (which may be streaked with blood)
- a lump or swelling in either of your armpits
- dimpling on the skin of your breasts
- a rash on or around your nipple
- a change in the appearance of your nipple, such as becoming sunken into your breast
Source: NHS